Introducing ciNMN®

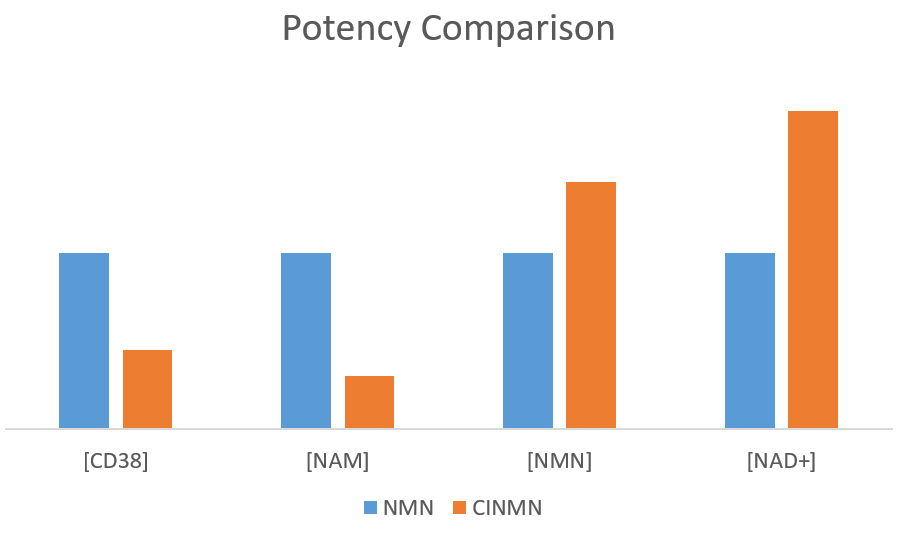
Long-term consumption of a single-ingredient Nicotinamide mononucleotide(NMN) has decayed health effects over time due to the face that NMN consumption induces Nicotinamide(NAM) accumulation. NAM accumulation has possible adverse effects.
Hwang ES, Song SB. Possible Adverse Effects of High-Dose Nicotinamide: Mechanisms and Safety Assessment. Biomolecules. 2020;10(5):687. Published 2020 Apr 29. doi:10.3390/biom10050687
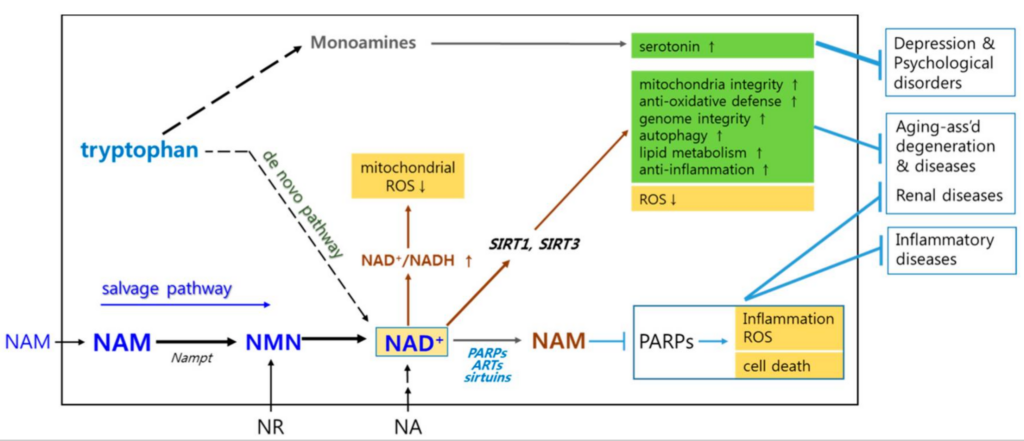
The synthesis of consumption and regeneration of NAD+ is a cycle involving intracellular and extracellular NMN→NAD+→NAM→NMN.
When NMN is supplemented alone, NMN will synthesize a large amount of NAD+ and be utilized by the body at first. However, when NAD+ completes its first round of functions, a large amount of NAM (nicotinamide) accumulates.
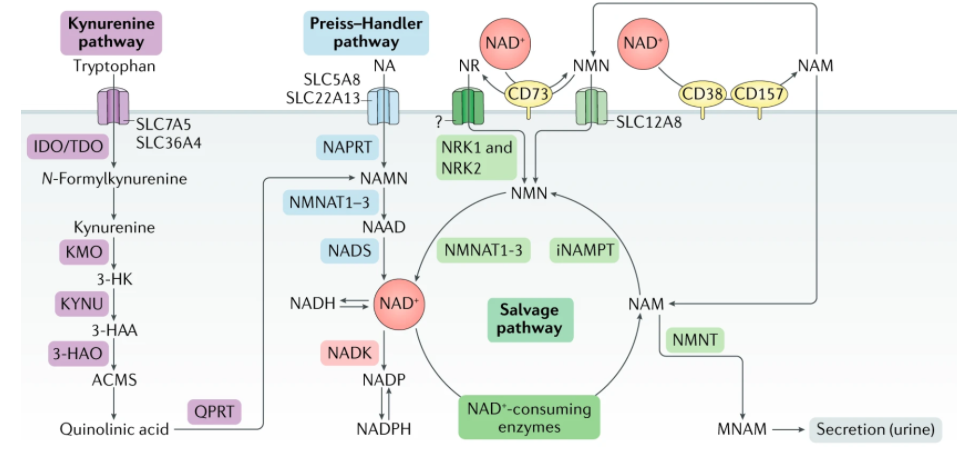
Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119-141. doi:10.1038/s41580-020-00313-x
Aged adults have reduced NAMPT functions and can no longer utilize as much NAM as before, resulting in lower overall circulation. On one hand, the NAM with excess capacity cannot be decomposed and reused. On the other hand, aging caused CD38 and CD157 accumulation in human body. These two type of glycoproteins consumes large amount of NAD+ for non-longevity functions, causing the longevity protein Sirtuin family and DNA repair enzymes unable to work properly.
CD38
CD38 (cluster of differentiation 38), also known as cyclic ADP ribose hydrolase, is a glycoprotein found on the surface of many immune cells (leukocytes), including CD4+, CD8+, B lymphocytes, and natural killer cells. CD38 also plays a role in cell adhesion, signal transduction and calcium signaling.
A gradual increase in CD38 is associated with a decline in NAD+ with age. Treating aged mice with specific CD38 inhibitors prevented the age-related decline in NAD+. CD38 knockout mice had twice the NAD+ levels and were resistant to age-related NAD+ declines, with significant increases in NAD+ levels in major organs (liver, muscle, brain and heart). Also, mice overexpressing CD38 exhibited reduced NAD+ and mitochondrial dysfunction.
Macrophages are thought to be responsible for the age-related increase in CD38 expression and decrease in NAD+. As we age, macrophages accumulate in visceral fat and other tissues, leading to chronic inflammation. The secretion of senescent cells induces high-level expression of CD38 on macrophages, which becomes the main cause of NAD+ senescence.
Nicotinamide ribose (NR) and nicotinamide mononucleotide (NMN) are NAD+ precursors, but when NR or NMN is administered, CD38 can degrade these precursors before they enter cells. Therefore, CD38 should be regulated so that NMN can play an anti-aging effect in the human body.
CD157 is a paralog of CD38, located on human chromosome 4 (4p15).
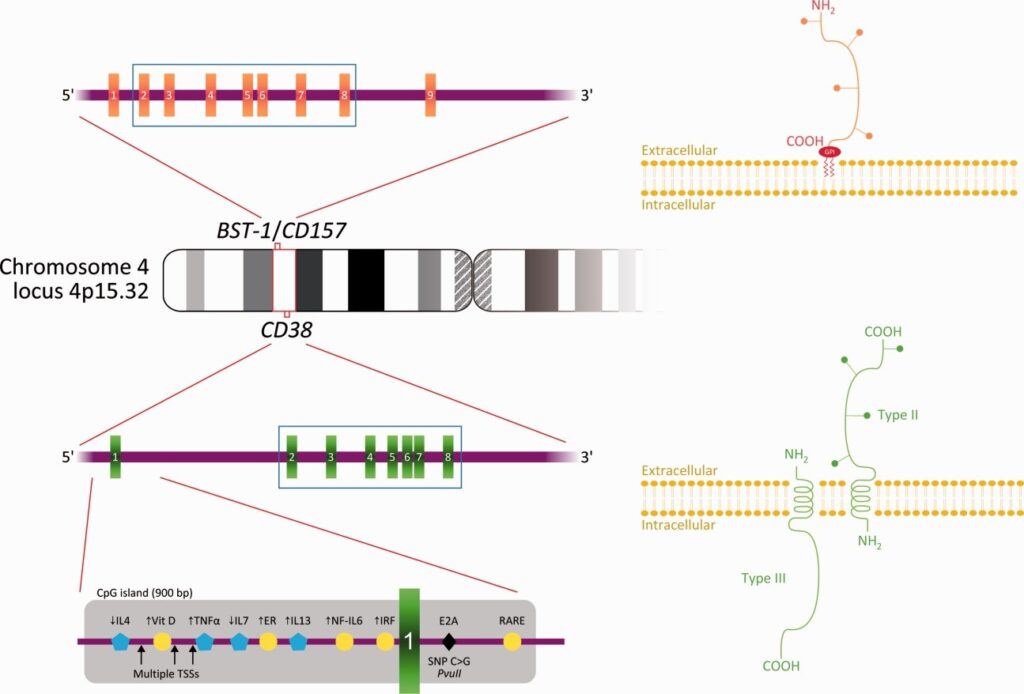
Schematic representation of human chromosome 4, note CD38 (and its regulatory elements) and CD157. Schematic representation of CD38 and CD157 membrane proteins is shown in the figure.
Both CD157 and CD38 are members of the ADP-ribosyl cyclase family, which catalyze the formation of NAD+ to form nicotinamide and adenosine diphosphate ribose (ADPR) or cyclic ADP-ribose (cADPR), with structural similarity, but CD157 has weaker activity than CD38. cADPR is required for the regulation of Ca2+ in cells. Only CD38 is able to hydrolyze cADPR to ADPR. CD38 is widely expressed in tissues, whereas CD157 is mainly found in intestinal and lymphoid tissues.
In summary, Single ingredient NMN cause NAM accumulation and CD38/CD157 reduces the beneficial effects of NMN.
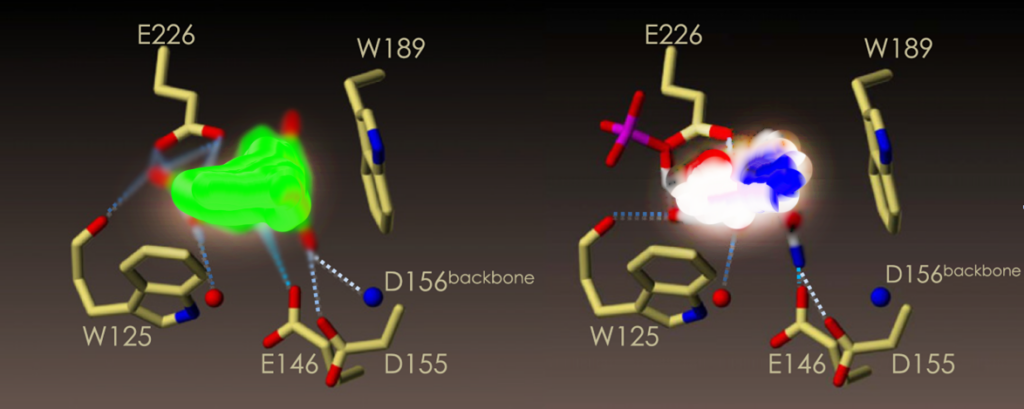
The model shows the binding of the active conformation of Q45-Q70 components to CD38/CD157. As shown, Q45-Q70 has the binding capacity to the hydrogen bond network of CD38 key site anchors (Trp125, Glu146 and Asp155) and residues (Glu226) of the enzymatic active site. Its interaction mode is similar to that observed in the X-ray crystal structure of the NMN+ moiety of the substrate NAD+.
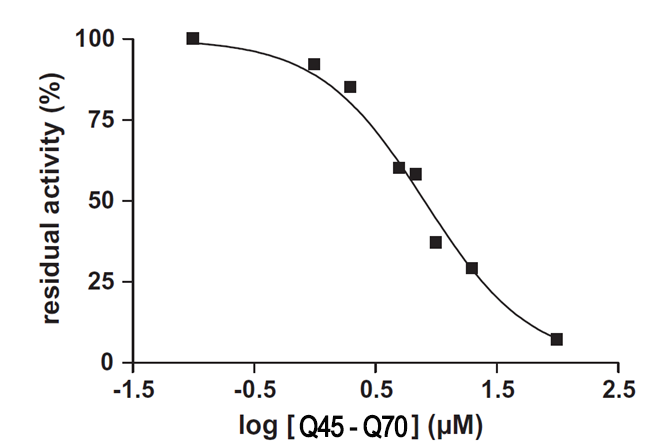
Concentration profile of Q45-Q70 components for inhibition of human CD38/CD157. Remaining enzymatic activity, expressed as a percentage, was plotted as a function of inhibitor logarithmic concentration using fluorescent labeling of 1,N6-etheno NAD+ as substrate and analyzed by nonlinear regression.
In summary: The Q45-Q70 components confirmed their inhibitory effects on CD38/CD157 from molecular simulation and experimental detection.
Eliminate obstacles for NMN to act in the human body.
Nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the rate-limiting step of the nicotinamide adenine dinucleotide (NAD+) salvage pathway. Activation of NAMPT is the key to recycle NAM as well as deduction of NAM concentration. After screening massive amount of potential fruit extract ingredients, a promising NAMPT activator is located. Combined with other ingredients, there is a significant drop of NAM level comparing with NAM level cause by single-ingredient NMN.
In summary: WithNAMPT activator and assisting ingredients, NAM level can decrease and the NMN beneficial effects can increase.
Conclusion
ciNMN® is a revolutionary material to inhibit CD38/CD157 activities to keep the beneficial effects of NMN, and to activate NAMPT for the consumption of NAM.